Website
www.eptri.eu
Role of CVBF
Project Coordinator
Description
ID-EPTRI project, coordinated by CVBF and funded within the H2020-INFRADEV-01-2017 programme, aims to create the framework for a new Research Infrastructure (RI) intended to enhance technology-driven paediatric research in drug discovery and early development phases to be translated into clinical research and paediatric use of medicines.
The project arises from the need to find answers to the serious lack of medicines for children in EU and worldwide and to propose development models for paediatric medicines that integrates technology-driven aspects with clinical trials. The interest for Paediatrics was indeed mentioned in the ESFRI Roadmap 2016 (http://www.esfri.eu/sites/default/files/20160309_ROADMAP_browsable.pdf) where it was recognised that a similar RI should be included into the landscape of the research in Europe.
EPTRI will be a complementary RI in the context of the existing RIs covering the current gaps in paediatric medicines. The new RI will represent a “paediatric common service” with three already established Research Infrastructures (BBMRI, EATRIS, ECRIN) to harness efficiency and delivery of paediatric research activities and services strengthening collaboration within the scientific paediatric community.
The final result of the project will be the Conceptual Design Report to realize EPTRI, the European Paediatric Translational Research Infrastructure, describing the scientific and technical requirements as well as the key components of the new RI.
To prepare the Conceptual Design Report (CDR), the project will encompass three phases.
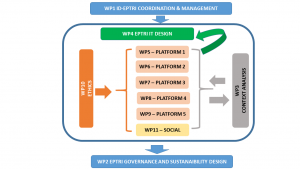 During the Context Analysis phase, that will be performed in 5 technical and scientific domains (1-Paediatric Medicines Discovery, 2- Biomarkers, 3-Paediatric Pharmacology, 4-Formulation Science, 5-Underpinning Paediatric Studies), the perceived value and the possible gaps to be covered will be estimated, by enquiring the scientific Communities, the concerned national Authorities and many other Stakeholders.
During the Context Analysis phase, that will be performed in 5 technical and scientific domains (1-Paediatric Medicines Discovery, 2- Biomarkers, 3-Paediatric Pharmacology, 4-Formulation Science, 5-Underpinning Paediatric Studies), the perceived value and the possible gaps to be covered will be estimated, by enquiring the scientific Communities, the concerned national Authorities and many other Stakeholders.
During the Operational phase, the different components of the new RI will be organised, including governance model, strategies for interaction with national Authorities and the existing RIs, the IT-architecture model, services to be provided and a business plan.
In the second year of the project, a Feasibility phase is proposed to develop virtual exercises simulating the operations of the RI, that will work as a “one-stop-shop” for advice in paediatric drug development in order to:
- support researchers in many methodological areas,
- foster the technology innovation in all the academic and clinical settings,
- conduct research that effectively underpins the development of medicines for children,
- increase the global effectiveness of the European research area also in favour of children and young people.
Funds
European Union’s Horizon 2020 programme
Budget
3.072 million euro
EU Contribution
3.00 million euro
Duration
2 years
Partners
(1) Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF), Italy; (2) Fondazione PENTA – For the treatment and care of children with HIV – ONLUS (PENTA), Italy; (3) European Infrastructure for Translational Medicine (EATRIS ERIC), The Netherlands; (4) University College London (UCL), United Kingdom; (5) ATHENA Research and innovation center in information communication & knowledge technologies (ATHENA), Greece; (6) Biobanks and BioMolecular resources Research Infrastructure Consortium (BBMRI-ERIC), Austria; (7) The Cyprus foundation for muscular dystrophy research (CING), Cyprus; (8) Assistance Publique – Hopitaux de Paris (AP-HP), France; (9) Stichting Katholieke Universiteit (RUMC), The Netherlands; (10) The University of Liverpool (ULIV), United Kingdom; (11) Romanian Angel Appeal Foundation (RAA), Romania; (12) Instytut Pomnik Centrum Zdrowia Dziecka (IPCZD), Poland; (13) Ospedale Pediatrico Bambino Gesù (OPBG), Italy; (14) Fyziologicky Ustav Akademie ved Ceske Republiky Verejna Vyzkumna Instituce (IPHYS), Czech Republic; (15) Fundacio Sant Joan de Deu (FSJD), Spain; (16) Nizhegorodskiy Gosudarstvenniy Universitet Im N.I. Lobachevskogo (UNN), Russia; (17) Servicio Madrileno de Salud (SERMAS-HULP), Spain; (18) European Clinical Research Infrastructure Network (ECRIN-ERIC), France; (19) Qendra Spitalore Universitare Nene Tereza Tirane (UHCT), Albania; (20) TECHNION – Israel Institute of Technology (TECHNION), Israel; (21) Tartu Ulikool (UTARTU), Estonia; (22) Universitatsklinikum Erlangen (UKER), Germany; (23) Swiss Clinical Trial Organisation Verein (SCTO), Switzerland; (24) Vastra Gotaland Lans Landsting (VGR), Sweden; (25) Universitair Medisch Centrum Utrecht (UMCU), The Netherlands; (26) State “Institute of Pediatrics Obstetrics and Gynecology National Academy of Medical Sciences of Ukraine” (UKR), Ukraine.
Other organizations linked to the project
Third parties involved in the project
Fondazione per la Ricerca Farmacologica Gianni Benzi Onlus “FGB”, Italy (CVBF third party); TEDDY European Network of Excellence for Paediatric Clinical Research, Italy (CVBF third party); Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Italy (CVBF third party); University of Bari Aldo Moro “UNIBA”, Italy (CVBF third party); Università degli Studi di Milano-Bicocca, Italy (BBMRI third party ); Fundación para la Investigación Biomédica del Hospital Universitario La Paz, Spain (SERMAS-HULP third party); Consorzio per Valutazioni Biologiche e Farmacologiche – Dege e shoqerise se huaj “CVBF-AL”, Albania (UHCT third party); Technion Research & Development Foundation Ltd. (“TRDF”), Israel, (TECHNION third party).
Supporting Organizations
Arthritis and Rheumatism Association Malta (ARAM), Malta; Asociación Española de Pediatría (AEP), Spain; Associação Nacional de Displasias Ósseas (ANDO), Portugal; Childhood Cancer International (CCI), The Netherlands; Cyprus Institute of Neurology and Genetics, Cyprus; Department of Paediatric Neurology, University Children’s Hospital Ljubljana, Slovenia; European Network for Rare and Congenital Anaemias (ENERCA), Spain; Eurordis, Rare Disease Europe, France; Foundation MÁS QUE IDEAS, Spain; Foundation of child neurology, Slovenia; Fundaciòn ALPE acondroplasia, Spain; Greek Carers Network – EPIONI, Greece; LIFE (ZIVOT) – Organization for children rare diseases, Serbia; Norwegian Melanoma Patient Association (Foflekkreftforeningen), Norway.